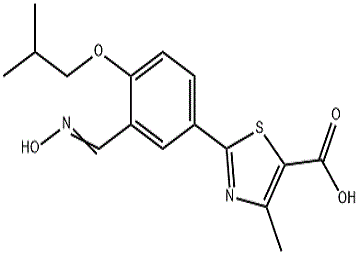
English Synonyms
FebuxostatImpurity24/(Z)-2-(3-((hydroxyimino)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylicacid;Febuxostat-17;FebuxostatImpurity7;(Z)-2-(3-((hydroxyimino)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylicacid;FebuxostatImpurityM;3-Descyano-3-((hydroxyimino)methyl)Febuxostat;FebuxostatImpurity7(F);2-(3-((Hydroxyimino)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylicacid
Febuxostat Impurity 7 Properties
| Melting Point | >215°C (dec.) |
| Storage Conditions | -20°C Freezer |
| Solubility | Dimethyl Sulfoxide (slightly soluble) |
| Form | Solid |
| Color | color |
Febuxostat Impurity 7 Usage and Synthesis
Application
Febuxostat Impurity 7 is an impurity produced during the synthesis of febuxostat and can also be used in laboratory research and development processes and chemical and pharmaceutical synthesis processes.
Preparation
2-[3-((Hydroxyimino)methyl)-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid (Febuxostat Impurity 7) Compound Preparation: Add 2-[3-((hydroxyimino)methyl)-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxy to pre-cooled sodium hydroxide. A solution of ethyl acid ester (100 g) in methanol (500 ml) (22.10 g in 500 ml water). The reaction mixture was heated to 75°C and stirred at the same temperature for 30 minutes. Water (5 g) was added to the reaction mixture and the reaction mixture was cooled to 25 °C. Methanol (100ml) and water (100ml) were added to the reaction mixture. Adjust the pH to 2.0-3.0 using aqueous hydrochloric acid solution. The reaction mixture was stirred for 5 hours. The precipitated solid was filtered and washed with methanol and water. The resulting material is dried. To the resulting material were added tetrahydrofuran (270ml) and carbon (2.25gms). The reaction mixture was stirred for 30 minutes and filtered through hyflow. The solvent was completely distilled from the filtrate. Methanol (450 ml) was added to the resulting compound and stirred at 60°C for 30 minutes. The reaction mixture was cooled to 25°C and stirred for 2 hours. The precipitated solid was filtered and the material dried to give the pure title compound Febuxostat Impurity 7. Yield: 70 g.